










Components:
Method of action:
Treatment option:
Medically reviewed by Fedorchenko Olga Valeryevna, PharmD. Last updated on 06.04.2022

Attention! Information on this page is intended only for medical professionals! Information is collected in open sources and may contain significant errors! Be careful and double-check all the information on this page!
Dosage Forms And Strengths
Extended-Release Tablets
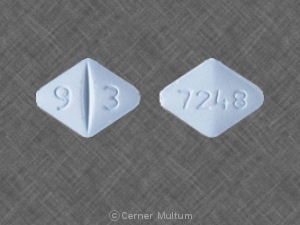
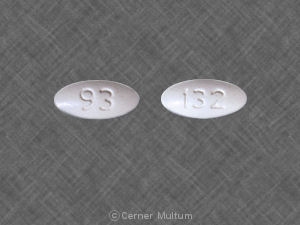
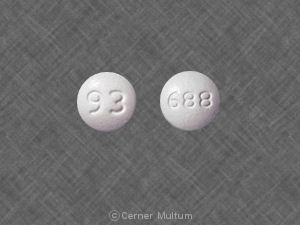
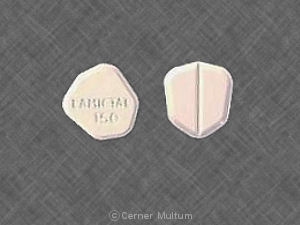
25 mg, yellow with white center, round, biconvex, film-coated tablets printed with “LAMICTAL” and “XR 25.”
50 mg, green with white center, round, biconvex, film-coated tablets printed with “LAMICTAL” and “XR 50.”
100 mg, orange with white center, round, biconvex, film-coated tablets printed with “LAMICTAL” and “XR 100.”
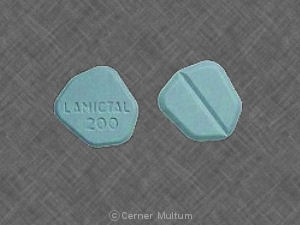
200 mg, blue with white center, round, biconvex, film-coated tablets printed with “LAMICTAL” and “XR 200.”
250 mg, purple with white center, caplet-shaped, film-coated tablets printed with “LAMICTAL” and “XR 250.”
300 mg, gray with white center, caplet-shaped, film-coated tablets printed with “LAMICTAL” and “XR 300.”
Storage And Handling
LAMICTAL XR (lamotrigine) Extended-release Tablets
25 mg, yellow with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 25”, unit-of-use bottles of 30 with orange caps (NDC 0173-0754-00).
50 mg, green with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 50”, unit-of-use bottles of 30 with orange caps (NDC 0173-0755-00).
100 mg, orange with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 100”, unit-of-use bottles of 30 with orange caps (NDC 0173-0756-00).
200 mg, blue with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 200”, unit-of-use bottles of 30 with orange caps (NDC 0173-0757-00).
250 mg, purple with a white center, caplet-shaped, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 250”, unit-of-use bottles of 30 with orange caps (NDC 0173-0781-00).
300 mg, gray with a white center, caplet-shaped, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 300”, unit-of-use bottles of 30 with orange caps (NDC 0173-0761-00).
LAMICTAL XR (lamotrigine) Patient Titration Kit for Patients Taking Valproate (Blue XR Kit)
25 mg, yellow with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 25” and 50 mg, green with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 50”; blisterpack of 21/25-mg tablets and 7/50-mg tablets (NDC 0173-0758-00).
LAMICTAL XR (lamotrigine) Patient Titration Kit for Patients Taking Carbamazepine, Phenytoin, Phenobarbital, or Primidone, and Not Taking Valproate (Green XR Kit)
50 mg, green with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 50”; 100 mg, orange with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 100”; and 200 mg, blue with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 200”; blisterpack of 14/50-mg tablets, 14/100-mg tablets, and 7/200-mg tablets (NDC 0173-0759-00).
LAMICTAL XR (lamotrigine) Patient Titration Kit for Patients Not Taking Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate (Orange XR Kit)
25 mg, yellow with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 25”; 50 mg, green with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 50”; and 100 mg, orange with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 100”; blisterpack of 14/25-mg tablets, 14/50-mg tablets, and 7/100-mg tablets (NDC 0173-0760-00).
Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
GlaxoSmithKline, Research Triangle Park, NC 27709 ©2015, the GSK group of companies. Revised: March 2015
Adjunctive Therapy
LAMICTAL XR is indicated as adjunctive therapy for primary generalized tonic-clonic (PGTC) seizures and partial-onset seizures with or without secondary generalization in patients aged 13 years and older.
Monotherapy
LAMICTAL XR is indicated for conversion to monotherapy in patients aged 13 years and older with partial-onset seizures who are receiving treatment with a single antiepileptic drug (AED).
Safety and effectiveness of LAMICTAL XR have not been established (1) as initial monotherapy or (2) for simultaneous conversion to monotherapy from 2 or more concomitant AEDs.
Limitation Of Use
Safety and effectiveness of LAMICTAL XR for use in patients younger than 13 years have not been established.
LAMICTAL XR extended-release tablets are taken once daily, with or without food. Tablets must be swallowed whole and must not be chewed, crushed, or divided.
General Dosing Considerations
Rash
There are suggestions, yet to be proven, that the risk of severe, potentially life-threatening rash may be increased by (1) coadministration of LAMICTAL XR with valproate, (2) exceeding the recommended initial dose of LAMICTAL XR, or (3) exceeding the recommended dose escalation for LAMICTAL XR. However, cases have occurred in the absence of these factors. Therefore, it is important that the dosing recommendations be followed closely.
The risk of nonserious rash may be increased when the recommended initial dose and/or the rate of dose escalation for LAMICTAL XR is exceeded and in patients with a history of allergy or rash to other AEDs.
LAMICTAL XR Patient Titration Kits provide LAMICTAL XR at doses consistent with the recommended titration schedule for the first 5 weeks of treatment, based upon concomitant medications, for patients with partial-onset seizures and are intended to help reduce the potential for rash. The use of LAMICTAL XR Patient Titration Kits is recommended for appropriate patients who are starting or restarting LAMICTAL XR.
It is recommended that LAMICTAL XR not be restarted in patients who discontinued due to rash associated with prior treatment with lamotrigine unless the potential benefits clearly outweigh the risks. If the decision is made to restart a patient who has discontinued LAMICTAL XR, the need to restart with the initial dosing recommendations should be assessed. The greater the interval of time since the previous dose, the greater consideration should be given to restarting with the initial dosing recommendations. If a patient has discontinued lamotrigine for a period of more than 5 half-lives, it is recommended that initial dosing recommendations and guidelines be followed. The half-life of lamotrigine is affected by other concomitant medications.
LAMICTAL XR Added to Drugs Known to Induce or Inhibit Glucuronidation
Because lamotrigine is metabolized predominantly by glucuronic acid conjugation, drugs that are known to induce or inhibit glucuronidation may affect the apparent clearance of lamotrigine. Drugs that induce glucuronidation include carbamazepine, phenytoin, phenobarbital, primidone, rifampin, estrogen-containing oral contraceptives, and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir. Valproate inhibits glucuronidation. For dosing considerations for LAMICTAL XR in patients on estrogen-containing contraceptives and atazanavir/ritonavir, see below and Table 5. For dosing considerations for LAMICTAL XR in patients on other drugs known to induce or inhibit glucuronidation, see Table 1 and Table 5.
Target Plasma Levels
A therapeutic plasma concentration range has not been established for lamotrigine. Dosing of LAMICTAL XR should be based on therapeutic response.
Women Taking Estrogen-Containing Oral Contraceptives
Starting LAMICTAL XR in Women Taking Estrogen-Containing Oral Contraceptives: Although estrogen-containing oral contraceptives have been shown to increase the clearance of lamotrigine , no adjustments to the recommended dose-escalation guidelines for LAMICTAL XR should be necessary solely based on the use of estrogen-containing oral contraceptives. Therefore, dose escalation should follow the recommended guidelines for initiating adjunctive therapy with LAMICTAL XR based on the concomitant AED or other concomitant medications (see Table 1). See below for adjustments to maintenance doses of LAMICTAL XR in women taking estrogen-containing oral contraceptives.
Adjustments to the Maintenance Dose of LAMICTAL XR in Women Taking Estrogen-Containing Oral Contraceptives:
- Taking Estrogen-Containing Oral Contraceptives: In women not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation , the maintenance dose of LAMICTAL XR will in most cases need to be increased by as much as 2-fold over the recommended target maintenance dose to maintain a consistent lamotrigine plasma level.
- Starting Estrogen-Containing Oral Contraceptives: In women taking a stable dose of LAMICTAL XR and not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation , the maintenance dose will in most cases need to be increased by as much as 2-fold to maintain a consistent lamotrigine plasma level. The dose increases should begin at the same time that the oral contraceptive is introduced and continue, based on clinical response, no more rapidly than 50 to 100 mg/day every week. Dose increases should not exceed the recommended rate (see Table 1) unless lamotrigine plasma levels or clinical response support larger increases. Gradual transient increases in lamotrigine plasma levels may occur during the week of inactive hormonal preparation (pill-free week), and these increases will be greater if dose increases are made in the days before or during the week of inactive hormonal preparation. Increased lamotrigine plasma levels could result in additional adverse reactions, such as dizziness, ataxia, and diplopia. If adverse reactions attributable to LAMICTAL XR consistently occur during the pill-free week, dose adjustments to the overall maintenance dose may be necessary. Dose adjustments limited to the pill-free week are not recommended. For women taking LAMICTAL XR in addition to carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation , no adjustment to the dose of LAMICTAL XR should be necessary.
- Stopping Estrogen-Containing Oral Contraceptives: In women not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation , the maintenance dose of LAMICTAL XR will in most cases need to be decreased by as much as 50% in order to maintain a consistent lamotrigine plasma level. The decrease in dose of LAMICTAL XR should not exceed 25% of the total daily dose per week over a 2-week period, unless clinical response or lamotrigine plasma levels indicate otherwise. In women taking LAMICTAL XR in addition to carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation , no adjustment to the dose of LAMICTAL XR should be necessary.
Women and Other Hormonal Contraceptive Preparations or Hormone Replacement Therapy
The effect of other hormonal contraceptive preparations or hormone replacement therapy on the pharmacokinetics of lamotrigine has not been systematically evaluated. It has been reported that ethinylestradiol, not progestogens, increased the clearance of lamotrigine up to 2-fold, and the progestin-only pills had no effect on lamotrigine plasma levels. Therefore, adjustments to the dosage of LAMICTAL XR in the presence of progestogens alone will likely not be needed.
Patients Taking Atazanavir/Ritonavir
While atazanavir/ritonavir does reduce the lamotrigine plasma concentration, no adjustments to the recommended dose-escalation guidelines for LAMICTAL XR should be necessary solely based on the use of atazanavir/ritonavir. Dose escalation should follow the recommended guidelines for initiating adjunctive therapy with LAMICTAL XR based on concomitant AED or other concomitant medications (see Tables 1 and 5). In patients already taking maintenance doses of LAMICTAL XR and not taking glucuronidation inducers, the dose of LAMICTAL XR may need to be increased if atazanavir/ritonavir is added, or decreased if atazanavir/ritonavir is discontinued.
Patients With Hepatic Impairment
Experience in patients with hepatic impairment is limited. Based on a clinical pharmacology study in 24 subjects with mild, moderate, and severe liver impairment , the following general recommendations can be made. No dosage adjustment is needed in patients with mild liver impairment. Initial, escalation, and maintenance doses should generally be reduced by approximately 25% in patients with moderate and severe liver impairment without ascites and 50% in patients with severe liver impairment with ascites. Escalation and maintenance doses may be adjusted according to clinical response.
Patients With Renal Impairment
Initial doses of LAMICTAL XR should be based on patients' concomitant medications (see Table 1); reduced maintenance doses may be effective for patients with significant renal impairment. Few patients with severe renal impairment have been evaluated during chronic treatment with immediate-release lamotrigine. Because there is inadequate experience in this population, LAMICTAL XR should be used with caution in these patients.
Discontinuation Strategy
For patients receiving LAMICTAL XR in combination with other AEDs, a re-evaluation of all AEDs in the regimen should be considered if a change in seizure control or an appearance or worsening of adverse reactions is observed.
If a decision is made to discontinue therapy with LAMICTAL XR, a step-wise reduction of dose over at least 2 weeks (approximately 50% per week) is recommended unless safety concerns require a more rapid withdrawal.
Discontinuing carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation should prolong the half-life of lamotrigine; discontinuing valproate should shorten the half-life of lamotrigine.
Adjunctive Therapy For Primary Generalized Tonic-Clonic And Partial-Onset Seizures
This section provides specific dosing recommendations for patients aged 13 years and older. Specific dosing recommendations are provided depending upon concomitant AEDs or other concomitant medications.
Table 1: Escalation Regimen for LAMICTAL XR in
Patients Aged 13 Years and Older
| In Patients TAKING Valproatea | In Patients NOT TAKING Carbamazepine, Phenytoin, Phenobarbital, Primidone,b or Valproatea | In Patients TAKING Carbamazepine, Phenytoin, Phenobarbital, or Primidoneb and NOT TAKING Valproatea | |
| Weeks 1 and 2 | 25 mg every other day | 25 mg every day | 50 mg every day |
| Weeks 3 and 4 | 25 mg every day | 50 mg every day | 100 mg every day |
| Week 5 | 50 mg every day | 100 mg every day | 200 mg every day |
| Week 6 | 100 mg every day | 150 mg every day | 300 mg every day |
| Week 7 | 150 mg every day | 200 mg every day | 400 mg every day |
| Maintenance range (week 8 and onward) | 200 to 250 mg every dayc | 300 to 400 mg every dayc | 400 to 600 mg every dayc |
| aValproate has been shown to inhibit
glucuronidation and decrease the apparent clearance of lamotrigine. bDrugs that induce lamotrigine glucuronidation and increase clearance, other than the specified antiepileptic drugs, include estrogen-containing oral contraceptives, rifampin, and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir. Dosing recommendations for oral contraceptives and the protease inhibitor atazanavir/ritonavir can be found in General Dosing Considerations. Patients on rifampin and the protease inhibitor lopinavir/ritonavir should follow the same dosing titration/maintenance regimen used with antiepileptic drugs that induce glucuronidation and increase clearance. c Dose increases at week 8 or later should not exceed 100 mg daily at weekly intervals. |
Conversion From Adjunctive Therapy To Monotherapy
The goal of the transition regimen is to attempt to maintain seizure control while mitigating the risk of serious rash associated with the rapid titration of LAMICTAL XR.
To avoid an increased risk of rash, the recommended maintenance dosage range of LAMICTAL XR as monotherapy is 250 to 300 mg given once daily.
The recommended initial dose and subsequent dose escalations for LAMICTAL XR should not be exceeded.
Conversion From Adjunctive Therapy With Carbamazepine, Phenytoin, Phenobarbital, or Primidone to Monotherapy With LAMICTAL XR
After achieving a dose of 500 mg/day of LAMICTAL XR using the guidelines in Table 1, the concomitant enzyme-inducing AED should be withdrawn by 20% decrements each week over a 4-week period. Two weeks after completion of withdrawal of the enzyme-inducing AED, the dosage of LAMICTAL XR may be decreased no faster than 100 mg/day each week to achieve the monotherapy maintenance dosage range of 250 to 300 mg/day.
The regimen for the withdrawal of the concomitant AED is based on experience gained in the controlled monotherapy clinical trial using immediate-release lamotrigine.
Conversion From Adjunctive Therapy With Valproate to Monotherapy With LAMICTAL XR
The conversion regimen involves the 4 steps outlined in Table 2.
Table 2: Conversion From
Adjunctive Therapy With Valproate to Monotherapy With LAMICTAL XR in Patients
Aged 13 Years and Older With Epilepsy
| LAMICTAL XR | Valproate | |
| Step 1 | Achieve a dose of 150 mg/day according to guidelines in Table 1. | Maintain established stable dose. |
| Step 2 | Maintain at 150 mg/day. | Decrease dose by decrements no greater than 500 mg/day/week to 500 mg/day and then maintain for 1 week. |
| Step 3 | Increase to 200 mg/day. | Simultaneously decrease to 250 mg/day and maintain for 1 week. |
| Step 4 | Increase to 250 or 300 mg/day. | Discontinue. |
Conversion From Adjunctive Therapy With Antiepileptic Drugs Other Than Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate to Monotherapy With LAMICTAL XR
After achieving a dosage of 250 to 300 mg/day of LAMICTAL XR using the guidelines in Table 1, the concomitant AED should be withdrawn by 20% decrements each week over a 4-week period. No adjustment to the monotherapy dose of LAMICTAL XR is needed.
Conversion From Immediate-Release Lamotrigine Tablets To LAMICTAL XR
Patients may be converted directly from immediate-release lamotrigine to LAMICTAL XR extended-release tablets. The initial dose of LAMICTAL XR should match the total daily dose of immediate-release lamotrigine. However, some subjects on concomitant enzyme-inducing agents may have lower plasma levels of lamotrigine on conversion and should be monitored.
Following conversion to LAMICTAL XR, all patients (but especially those on drugs that induce lamotrigine glucuronidation) should be closely monitored for seizure control. Depending on the therapeutic response after conversion, the total daily dose may need to be adjusted within the recommended dosing instructions (Table 1).
The apparent clearance of lamotrigine is affected by the coadministration of certain medications.
The net effects of drug interactions with lamotrigine, based on drug interaction studies using immediate-release lamotrigine, are summarized in Tables 5 and 8, followed by details of the drug interaction studies below.
Table 8: Summary of Drug
Interactions With Lamotrigine
| Drug | Drug Plasma Concentration With Adjunctive Lamotriginea | Lamotrigine Plasma Concentration With Adjunctive Drugsb |
| Oral contraceptives (e.g., ethinylestradiol/levonorgestrel)c | ↔d | ↓ |
| Aripiprazole | Not assessed | ↔e |
| Atazanavir/ritonavir | ↔f | ↓ |
| Bupropion | Not assessed | ↔ |
| Carbamazepine | ↔ | ↓ |
| Carbamazepine epoxideg | ? | |
| Felbamate | Not assessed | ↔ |
| Gabapentin | Not assessed | ↔ |
| Levetiracetam | ↔ | ↔ |
| Lithium | ↔ | Not assessed |
| Lopinavir/ritonavir | ↔e | ↓ |
| Olanzapine | ↔ | ↔e |
| Oxcarbazepine | ↔ | ↔ |
| 10-Monohydroxy oxcarbazepine metaboliteh | ↔ | |
| Phenobarbital/primidone | ↔ | ↓ |
| Phenytoin | ↔ | ↓ |
| Pregabalin | ↔ | ↔ |
| Rifampin | Not assessed | ↓ |
| Risperidone | ↔ | Not assessed |
| 9-hydroxyrisperidonei | ↔ | |
| Topiramate | ↔j | ↔ |
| Valproate | ↓ | ↑ |
| Valproate + phenytoin and/or carbamazepine | Not assessed | ↔ |
| Zonisamide | Not assessed | ↔ |
| aFrom adjunctive clinical trials and volunteer
trials. bNet effects were estimated by comparing the mean clearance values obtained in adjunctive clinical trials and volunteer trials. c The effect of other hormonal contraceptive preparations or hormone replacement therapy on the pharmacokinetics of lamotrigine has not been systematically evaluated in clinical trials, although the effect may be similar to that seen with the ethinylestradiol/levonorgestrel combinations. dModest decrease in levonorgestrel. eSlight decrease, not expected to be clinically meaningful. fCompared to historical controls. gNot administered, but an active metabolite of carbamazepine. hNot administered, but an active metabolite of oxcarbazepine. iNot administered, but an active metabolite of risperidone. j Slight increase, not expected to be clinically meaningful. ↔ = No significant effect. ? = Conflicting data. |
Estrogen-Containing Oral Contraceptives
In 16 female volunteers, an oral contraceptive preparation containing 30 mcg ethinylestradiol and 150 mcg levonorgestrel increased the apparent clearance of lamotrigine (300 mg/day) by approximately 2-fold with mean decreases in AUC of 52% and in Cmax of 39%. In this study, trough serum lamotrigine concentrations gradually increased and were approximately 2-fold higher on average at the end of the week of the inactive hormone preparation compared with trough lamotrigine concentrations at the end of the active hormone cycle.
Gradual transient increases in lamotrigine plasma levels (approximate 2-fold increase) occurred during the week of inactive hormone preparation (pill-free week) for women not also taking a drug that increased the clearance of lamotrigine (carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation). The increase in lamotrigine plasma levels will be greater if the dose of LAMICTAL XR is increased in the few days before or during the pill-free week. Increases in lamotrigine plasma levels could result in dose-dependent adverse reactions.
In the same study, coadministration of lamotrigine (300 mg/day) in 16 female volunteers did not affect the pharmacokinetics of the ethinylestradiol component of the oral contraceptive preparation. There were mean decreases in the AUC and Cmax of the levonorgestrel component of 19% and 12%, respectively. Measurement of serum progesterone indicated that there was no hormonal evidence of ovulation in any of the 16 volunteers, although measurement of serum FSH, LH, and estradiol indicated that there was some loss of suppression of the hypothalamic-pituitary-ovarian axis.
The effects of doses of lamotrigine other than 300 mg/day have not been systematically evaluated in controlled clinical trials.
The clinical significance of the observed hormonal changes on ovulatory activity is unknown. However, the possibility of decreased contraceptive efficacy in some patients cannot be excluded. Therefore, patients should be instructed to promptly report changes in their menstrual pattern (e.g., break-through bleeding).
Dosage adjustments may be necessary for women receiving estrogen-containing oral contraceptive preparations.
Other Hormonal Contraceptives or Hormone Replacement Therapy
The effect of other hormonal contraceptive preparations or hormone replacement therapy on the pharmacokinetics of lamotrigine has not been systematically evaluated. It has been reported that ethinylestradiol, not progestogens, increased the clearance of lamotrigine up to 2-fold, and the progestin-only pills had no effect on lamotrigine plasma levels. Therefore, adjustments to the dosage of LAMICTAL XR in the presence of progestogens alone will likely not be needed.
Aripiprazole
In 18 patients with bipolar disorder on a stable regimen of 100 to 400 mg/day of lamotrigine, the lamotrigine AUC and Cmax were reduced by approximately 10% in patients who received aripiprazole 10 to 30 mg/day for 7 days, followed by 30 mg/day for an additional 7 days. This reduction in lamotrigine exposure is not considered clinically meaningful.
Atazanavir/Ritonavir
In a study in healthy volunteers, daily doses of atazanavir/ritonavir (300 mg/100 mg) reduced the plasma AUC and Cmax of lamotrigine (single 100-mg dose) by an average of 32% and 6%, respectively, and shortened the elimination half-lives by 27%. In the presence of atazanavir/ritonavir (300 mg/100 mg), the metabolite-to-lamotrigine ratio was increased from 0.45 to 0.71 consistent with induction of glucuronidation. The pharmacokinetics of atazanavir/ritonavir were similar in the presence of concomitant lamotrigine to the historical data of the pharmacokinetics in the absence of lamotrigine.
Bupropion
The pharmacokinetics of a 100-mg single dose of lamotrigine in healthy volunteers (n = 12) were not changed by coadministration of bupropion sustained-release formulation (150 mg twice daily) starting 11 days before lamotrigine.
Carbamazepine
Lamotrigine has no appreciable effect on steady-state carbamazepine plasma concentration. Limited clinical data suggest there is a higher incidence of dizziness, diplopia, ataxia, and blurred vision in patients receiving carbamazepine with lamotrigine than in patients receiving other AEDs with lamotrigine. The mechanism of this interaction is unclear. The effect of lamotrigine on plasma concentrations of carbamazepine-epoxide is unclear. In a small subset of patients (n = 7) studied in a placebo-controlled trial, lamotrigine had no effect on carbamazepine-epoxide plasma concentrations, but in a small, uncontrolled study (n = 9), carbamazepine-epoxide levels increased.
The addition of carbamazepine decreases lamotrigine steady-state concentrations by approximately 40%.
Esomeprazole
In a study of 30 subjects, coadministration of LAMICTAL XR with esomeprazole resulted in no significant change in lamotrigine levels and a small decrease in Tmax. The levels of gastric pH were not altered compared with pre-lamotrigine dosing.
Felbamate
In a trial in 21 healthy volunteers, coadministration of felbamate (1,200 mg twice daily) with lamotrigine (100 mg twice daily for 10 days) appeared to have no clinically relevant effects on the pharmacokinetics of lamotrigine.
Folate Inhibitors
Lamotrigine is a weak inhibitor of dihydrofolate reductase. Prescribers should be aware of this action when prescribing other medications that inhibit folate metabolism.
Gabapentin
Based on a retrospective analysis of plasma levels in 34 subjects who received lamotrigine both with and without gabapentin, gabapentin does not appear to change the apparent clearance of lamotrigine.
Levetiracetam
Potential drug interactions between levetiracetam and lamotrigine were assessed by evaluating serum concentrations of both agents during placebo-controlled clinical trials. These data indicate that lamotrigine does not influence the pharmacokinetics of levetiracetam and that levetiracetam does not influence the pharmacokinetics of lamotrigine.
Lithium
The pharmacokinetics of lithium were not altered in healthy subjects (n = 20) by coadministration of lamotrigine (100 mg/day) for 6 days.
Lopinavir/Ritonavir
The addition of lopinavir (400 mg twice daily)/ritonavir (100 mg twice daily) decreased the AUC, Cmax, and elimination half-life of lamotrigine by approximately 50% to 55.4% in 18 healthy subjects. The pharmacokinetics of lopinavir/ritonavir were similar with concomitant lamotrigine, compared to that in historical controls.
Olanzapine
The AUC and Cmax of olanzapine were similar following the addition of olanzapine (15 mg once daily) to lamotrigine (200 mg once daily) in healthy male volunteers (n = 16) compared with the AUC and Cmax in healthy male volunteers receiving olanzapine alone (n = 16).
In the same trial, the AUC and Cmax of lamotrigine were reduced on average by 24% and 20%, respectively, following the addition of olanzapine to lamotrigine in healthy male volunteers compared with those receiving lamotrigine alone. This reduction in lamotrigine plasma concentrations is not expected to be clinically meaningful.
Oxcarbazepine
The AUC and Cmax of oxcarbazepine and its active 10-monohydroxy oxcarbazepine metabolite were not significantly different following the addition of oxcarbazepine (600 mg twice daily) to lamotrigine (200 mg once daily) in healthy male volunteers (n = 13) compared with healthy male volunteers receiving oxcarbazepine alone (n = 13).
In the same trial, the AUC and Cmax of lamotrigine were similar following the addition of oxcarbazepine (600 mg twice daily) to lamotrigine in healthy male volunteers compared with those receiving lamotrigine alone. Limited clinical data suggest a higher incidence of headache, dizziness, nausea, and somnolence with coadministration of lamotrigine and oxcarbazepine compared with lamotrigine alone or oxcarbazepine alone.
Phenobarbital, Primidone
The addition of phenobarbital or primidone decreases lamotrigine steady-state concentrations by approximately 40%.
Phenytoin
Lamotrigine has no appreciable effect on steady-state phenytoin plasma concentrations in patients with epilepsy. The addition of phenytoin decreases lamotrigine steady-state concentrations by approximately 40%.
Pregabalin
Steady-state trough plasma concentrations of lamotrigine were not affected by concomitant pregabalin (200 mg 3 times daily) administration. There are no pharmacokinetic interactions between lamotrigine and pregabalin.
Rifampin
In 10 male volunteers, rifampin (600 mg/day for 5 days) significantly increased the apparent clearance of a single 25-mg dose of lamotrigine by approximately 2-fold (AUC decreased by approximately 40%).
Risperidone
In a 14 healthy volunteers study, multiple oral doses of lamotrigine 400 mg daily had no clinically significant effect on the single-dose pharmacokinetics of risperidone 2 mg and its active metabolite 9-OH risperidone. Following the coadministration of risperidone 2 mg with lamotrigine, 12 of the 14 volunteers reported somnolence compared with 1 out of 20 when risperidone was given alone, and none when lamotrigine was administered alone.
Topiramate
Topiramate resulted in no change in plasma concentrations of lamotrigine. Administration of lamotrigine resulted in a 15% increase in topiramate concentrations.
Valproate
When lamotrigine was administered to healthy volunteers (n = 18) receiving valproate, the trough steady-state valproate plasma concentrations decreased by an average of 25% over a 3-week period, and then stabilized. However, adding lamotrigine to the existing therapy did not cause a change in valproate plasma concentrations in either adult or pediatric patients in controlled clinical trials.
The addition of valproate increased lamotrigine steady-state concentrations in normal volunteers by slightly more than 2-fold. In 1 trial, maximal inhibition of lamotrigine clearance was reached at valproate doses between 250 and 500 mg/day and did not increase as the valproate dose was further increased.
Zonisamide
In a study in 18 patients with epilepsy, coadministration of zonisamide (200 to 400 mg/day) with lamotrigine (150 to 500 mg/day for 35 days) had no significant effect on the pharmacokinetics of lamotrigine.
Known Inducers or Inhibitors of Glucuronidation
Drugs other than those listed above have not been systematically evaluated in combination with lamotrigine. Since lamotrigine is metabolized predominately by glucuronic acid conjugation, drugs that are known to induce or inhibit glucuronidation may affect the apparent clearance of lamotrigine, and doses of LAMICTAL XR may require adjustment based on clinical response.
Other
In vitro assessment of the inhibitory effect of lamotrigine at OCT2 demonstrate that lamotrigine, but not the N(2)-glucuronide metabolite, is an inhibitor of OCT2 at potentially clinically relevant concentrations, with IC50 value of 53.8 μM.
Results of in vitro experiments suggest that clearance of lamotrigine is unlikely to be reduced by concomitant administration of amitriptyline, clonazepam, clozapine, fluoxetine, haloperidol, lorazepam, phenelzine, sertraline, or trazodone.
Results of in vitro experiments suggest that lamotrigine does not reduce the clearance of drugs eliminated predominantly by CYP2D6.
Teratogenic Effects
Pregnancy Category C. There are no adequate and well-controlled trials in pregnant women. In animal studies, lamotrigine was developmentally toxic at doses lower than those administered clinically. LAMICTAL XR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
When lamotrigine was administered to pregnant mice, rats, or rabbits during the period of organogenesis (oral doses of up to 125, 25, and 30 mg/kg, respectively), reduced fetal body weight and increased incidences of fetal skeletal variations were seen in mice and rats at doses that were also maternally toxic. The no-effect doses for embryo-fetal developmental toxicity in mice, rats, and rabbits (75, 6.25, and 30 mg/kg, respectively) are similar to (mice and rabbits) or less than the human dose of 400 mg/day on a body surface area (mg/m²) basis.
In a study in which pregnant rats were administered lamotrigine (oral doses of 5 or 25 mg/kg) during the period of organogenesis and offspring were evaluated postnatally, behavioral abnormalities were observed in exposed offspring at both doses. The lowest effect dose for developmental neurotoxicity in rats is less than the human dose of 400 mg/day on a mg/m² basis. Maternal toxicity was observed at the higher dose tested.
When pregnant rats were administered lamotrigine (oral doses of 5, 10, or 20 mg/kg) during the latter part of gestation, increased offspring mortality (including stillbirths) was seen at all doses. The lowest effect dose for peri/postnatal developmental toxicity in rats is less than the human dose of 400 mg/day on a mg/m² basis. Maternal toxicity was observed at the 2 highest doses tested.
Lamotrigine decreases fetal folate concentrations in rat, an effect known to be associated with adverse pregnancy outcomes in animals and humans.
Nonteratogenic Effects
As with other AEDs, physiological changes during pregnancy may affect lamotrigine concentrations and/or therapeutic effect. There have been reports of decreased lamotrigine concentrations during pregnancy and restoration of pre-partum concentrations after delivery. Dosage adjustments may be necessary to maintain clinical response.
Pregnancy Registry
To provide information regarding the effects of in utero exposure to LAMICTAL XR, physicians are advised to recommend that pregnant patients taking LAMICTAL XR enroll in the North American Antiepileptic Drug (NAAED)
Pregnancy Registry
This can be done by calling the toll-free number 1-888-233-2334 and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregi stry.org.
The following adverse reactions are described in more detail in the WARNINGS AND PRECAUTIONS section of the label:
- Serious skin rashes
- Multiorgan hypersensitivity reactions and organ failure
- Blood dyscrasias
- Suicidal behavior and ideation
- Aseptic meningitis
- Withdrawal seizures
- Status epilepticus
- Sudden unexplained death in epilepsy
Clinical Trial Experience With LAMICTAL XR For Treatment Of Primary Generalized Tonic-Clonic And Partial-Onset Seizures
Most Common Adverse Reactions In Clinical Trials
Adjunctive Therapy in Patients With Epilepsy: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In these 2 trials, adverse reactions led to withdrawal of 4 (2%) patients in the group receiving placebo and 10 (5%) patients in the group receiving LAMICTAL XR. Dizziness was the most common reason for withdrawal in the group receiving LAMICTAL XR (5 patients [3%]). The next most common adverse reactions leading to withdrawal in 2 patients each (1%) were rash, headache, nausea, and nystagmus.
Table 4 displays the incidence of adverse reactions in these two 19-week, double-blind, placebo-controlled trials of patients with PGTC and partial onset seizures.
Table 4: Adverse Reactions in Pooled,
Placebo-Controlled, Adjunctive Trials in Patients With Epilepsya
| Body System/ Adverse Reaction |
Percent of Patients Receiving Adjunctive LAMICTAL XR (n = 190) |
Percent of Patients Receiving Adjunctive Placebo (n = 195) |
| Ear and labyrinth disorders | ||
| Vertigo | 3 | < 1 |
| Eye disorders | ||
| Diplopia | 5 | < 1 |
| Vision blurred | 3 | 2 |
| Gastrointestinal disorders | ||
| Nausea | 7 | 4 |
| Vomiting | 6 | 3 |
| Diarrhea | 5 | 3 |
| Constipation | 2 | < 1 |
| Dry mouth | 2 | 1 |
| General disorders and administration site conditions | ||
| Asthenia and fatigue | 6 | 4 |
| Infections and infestations | ||
| Sinusitis | 2 | 1 |
| Metabolic and nutritional disorders | ||
| Anorexia | 3 | 2 |
| Musculoskeletal and connective tissue disorder | ||
| Myalgia | 2 | 0 |
| Nervous system | ||
| Dizziness | 14 | 6 |
| Tremor and intention tremor | 6 | 1 |
| Somnolence | 5 | 3 |
| Cerebellar coordination and balance disorder | 3 | 0 |
| Nystagmus | 2 | < 1 |
| Psychiatric disorders | ||
| Depression | 3 | < 1 |
| Anxiety | 3 | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||
| Pharyngolaryngeal pain | 3 | 2 |
| Vascular disorder | ||
| Hot flush | 2 | 0 |
| aAdverse reactions that occurred in at least 2% of patients treated with LAMICTAL XR and at a greater incidence than placebo. | ||
Note: In these trials the incidence of nonserious rash was 2% for LAMICTAL XR and 3% for placebo. In clinical trials evaluating immediate-release lamotrigine, the rate of serious rash was 0.3% in adults on adjunctive therapy for epilepsy.
Adverse reactions were also analyzed to assess the incidence of the onset of an event in the titration period, and in the maintenance period, and if adverse reactions occurring in the titration phase persisted in the maintenance phase.
The incidence for many adverse reactions caused by treatment with LAMICTAL XR was increased relative to placebo (i.e., treatment difference between LAMICTAL XR and placebo ≥ 2%) in either the titration or maintenance phases of the trial. During the titration phase, an increased incidence (shown in descending order of % treatment difference) was observed for diarrhea, nausea, vomiting, somnolence, vertigo, myalgia, hot flush, and anxiety. During the maintenance phase, an increased incidence was observed for dizziness, tremor, and diplopia. Some adverse reactions developing in the titration phase were notable for persisting ( > 7 days) into the maintenance phase. These persistent adverse reactions included somnolence and dizziness.
There were inadequate data to evaluate the effect of dose and/or concentration on the incidence of adverse reactions because, although patients were randomized to different target doses based upon concomitant AEDs, the plasma exposure was expected to be generally similar among all patients receiving different doses. However, in a randomized, parallel trial comparing placebo with 300 and 500 mg/day of immediate-release lamotrigine, the incidence of the most common adverse reactions ( ≥ 5%) such as ataxia, blurred vision, diplopia, and dizziness were dose related. Less common adverse reactions ( < 5%) were not assessed for dose-response relationships.
Monotherapy in Patients With Epilepsy: Adverse reactions observed in this trial were generally similar to those observed and attributed to drug in adjunctive and monotherapy immediate-release lamotrigine and adjunctive LAMICTAL XR placebo-controlled trials. Only 2 adverse events, nasopharyngitis and upper respiratory tract infection, were observed at a rate of ≥ 3% and not reported at a similar rate in previous trials. Because this trial did not include a placebo control group, causality could not be established.
Other Adverse Reactions Observed During The Clinical Development Of Immediate-Release Lamotrigine
All reported reactions are included except those already listed in the previous tables or elsewhere in the labeling, those too general to be informative, and those not reasonably associated with the use of the drug.
Adjunctive Therapy in Adults With Epilepsy
In addition to the adverse reactions reported above from the development of LAMICTAL XR, the following adverse reactions with an uncertain relationship to lamotrigine were reported during the clinical development of immediate-release lamotrigine for treatment of epilepsy in adults. These reactions occurred in ≥ 2% of patients receiving immediate-release lamotrigine and more frequently than in the placebo group.
Body as a Whole: Headache, flu syndrome, fever, neck pain.
Musculoskeletal: Arthralgia.
Nervous: Insomnia, convulsion, irritability, speech disorder, concentration disturbance.
Respiratory: Pharyngitis, cough increased.
Skin and Appendages: Rash, pruritus.
Urogenital (female patients only): Vaginitis, amenorrhea, dysmenorrhea.
Monotherapy in Adults With Epilepsy
In addition to the adverse reactions reported above from the development of LAMICTAL XR, the following adverse reactions with an uncertain relationship to lamotrigine were reported during the clinical development of immediate-release lamotrigine for treatment of epilepsy in adults. These reactions occurred in > 2% of patients receiving immediate-release lamotrigine and more frequently than in the placebo group.
Body as a Whole: Chest pain.
Digestive: Rectal hemorrhage, peptic ulcer.
Metabolic and Nutritional: Weight decrease, peripheral edema.
Nervous: Hypesthesia, libido increase, decreased reflexes.
Respiratory: Epistaxis, dyspnea.
Skin and Appendages: Contact dermatitis, dry skin, sweating.
Special Senses: Vision abnormality.
Urogenital (female patients only): Dysmenorrhea.
Other Clinical Trial Experience
Immediate-release lamotrigine has been administered to 6,694 individuals for whom complete adverse reaction data was captured during all clinical trials, only some of which were placebo controlled.
Adverse reactions are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse reactions are defined as those occurring in at least 1/100 patients; Infrequent adverse reactions are those occurring in 1/100 to 1/1,000 patients; rare adverse reactions are those occurring in fewer than 1/1,000 patients.
Cardiovascular System: Infrequent: Hypertension, palpitations, postural hypotension, syncope, tachycardia, vasodilation.
Dermatological: Infrequent: Acne, alopecia, hirsutism, maculopapular rash, urticaria. Rare: Leukoderma, multiforme erythema, petechial rash, pustular rash.
Digestive System: Infrequent: Dysphagia, liver function tests abnormal, mouth ulceration. Rare: Gastrointestinal hemorrhage, hemorrhagic colitis, hepatitis, melena and stomach ulcer.
Endocrine System: Rare: Goiter, hypothyroidism.
Hematologic and Lymphatic System: Infrequent: Ecchymosis, leukopenia. Rare: Anemia, eosinophilia, fibrin decrease, fibrinogen decrease, iron deficiency anemia, leukocytosis, lymphocytosis, macrocytic anemia, petechia, thrombocytopenia.
Metabolic and Nutritional Disorders: Infrequent: Aspartate transaminase increased. Rare: Alcohol intolerance, alkaline phosphatase increase, alanine transaminase increase, bilirubinemia, gamma glutamyl transpeptidase increase, hyperglycemia.
Musculoskeletal System: Rare: Muscle atrophy, pathological fracture, tendinous contracture.
Nervous System: Frequent: Confusion.
Infrequent: Akathisia, apathy, aphasia, depersonalization, dysarthria, dyskinesia, euphoria, hallucinations, hostility, hyperkinesia, hypertonia, libido decreased, memory decrease, mind racing, movement disorder, myoclonus, panic attack, paranoid reaction, personality disorder, psychosis, stupor. Rare: Choreoathetosis, delirium, delusions, dysphoria, dystonia, extrapyramidal syndrome, hemiplegia, hyperalgesia, hyperesthesia, hypokinesia, hypotonia, manic depression reaction, neuralgia, paralysis, peripheral neuritis.
Respiratory System: Rare: Hiccup, hyperventilation.
Special Senses: Frequent: Amblyopia. Infrequent: Abnormality of accommodation, conjunctivitis, dry eyes, ear pain, photophobia, taste perversion, tinnitus. Rare: Deafness, lacrimation disorder, oscillopsia, parosmia, ptosis, strabismus, taste loss, uveitis, visual field defect.
Urogenital System: Infrequent: Abnormal ejaculation, hematuria, impotence, menorrhagia, polyuria, urinary incontinence. Rare: Acute kidney failure, breast neoplasm, creatinine increase, female lactation, kidney failure, kidney pain, nocturia, urinary retention, urinary urgency.
Postmarketing Experience With Immediate-Release Lamotrigine
The following adverse events (not listed above in clinical trials or other sections of the prescribing information) have been identified during postapproval use of immediate-release lamotrigine. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic
Agranulocytosis, hemolytic anemia, lymphadenopathy not associated with hypersensitivity disorder.
Gastrointestinal
Esophagitis.
Hepatobiliary Tract and Pancreas
Pancreatitis.
Immunologic
Lupus-like reaction, vasculitis.
Lower Respiratory
Apnea.
Musculoskeletal
Rhabdomyolysis has been observed in patients experiencing hypersensitivity reactions.
Nervous System
Aggression, exacerbation of Parkinsonian symptoms in patients with pre-existing Parkinson's disease, tics.
Non-site Specific
Progressive immunosuppression.
Folate Metabolism
In vitro, lamotrigine inhibited dihydrofolate reductase, the enzyme that catalyzes the reduction of dihydrofolate to tetrahydrofolate. Inhibition of this enzyme may interfere with the biosynthesis of nucleic acids and proteins. When oral daily doses of lamotrigine were given to pregnant rats during organogenesis, fetal, placental, and maternal folate concentrations were reduced. Significantly reduced concentrations of folate are associated with teratogenesis. Folate concentrations were also reduced in male rats given repeated oral doses of lamotrigine. Reduced concentrations were partially returned to normal when supplemented with folinic acid.
Cardiovascular
In dogs, lamotrigine is extensively metabolized to a 2-N-methyl metabolite. This metabolite causes dose-dependent prolongation of the PR interval, widening of the QRS complex, and, at higher doses, complete AV conduction block. Similar cardiovascular effects are not anticipated in humans because only trace amounts of the 2-N-methyl metabolite ( < 0.6% of lamotrigine dose) have been found in human urine. However, it is conceivable that plasma concentrations of this metabolite could be increased in patients with a reduced capacity to glucuronidate lamotrigine (e.g., in patients with liver disease, patients taking concomitant medications that inhibit glucuronidation).
In comparison with immediate-release lamotrigine, the plasma lamotrigine levels following administration of LAMICTAL XR are not associated with any significant changes in trough plasma concentrations, and are characterized by lower peaks, longer time to peaks, and lower peak-to-trough fluctuation, as described in detail below.
Absorption
Lamotrigine is absorbed after oral administration with negligible first-pass metabolism. The bioavailability of lamotrigine is not affected by food.
In an open-label, crossover study of 44 subjects with epilepsy receiving concomitant AEDs, the steady-state pharmacokinetics of lamotrigine were compared following administration of equivalent total doses of LAMICTAL XR given once daily with those of lamotrigine immediate-release given twice daily. In this study, the median time to peak concentration (Tmax) following administration of LAMICTAL XR was 4 to 6 hours in subjects taking carbamazepine, phenytoin, phenobarbital, or primidone; 9 to 11 hours in subjects taking valproate; and 6 to 10 hours in subjects taking AEDs other than carbamazepine, phenytoin, phenobarbital, primidone, or valproate. In comparison, the median Tmax following administration of immediate-release lamotrigine was between 1 and 1.5 hours.
The steady-state trough concentrations for extended-release lamotrigine were similar to or higher than those of immediate-release lamotrigine depending on concomitant AED (Table 6). A mean reduction in the lamotrigine Cmax by 11% to 29% was observed for LAMICTAL XR compared with immediate-release lamotrigine, resulting in a decrease in the peak-to-trough fluctuation in serum lamotrigine concentrations. However, in some subjects receiving enzyme-inducing AEDs, a reduction in Cmax of 44% to 77% was observed. The degree of fluctuation was reduced by 17% in subjects taking enzyme-inducing AEDs; 34% in subjects taking valproate; and 37% in subjects taking AEDs other than carbamazepine, phenytoin, phenobarbital, primidone, or valproate. LAMICTAL XR and immediate-release lamotrigine regimens were similar with respect to area under the curve (AUC, a measure of the extent of bioavailability) for subjects receiving AEDs other than those known to induce the metabolism of lamotrigine. The relative bioavailability of extended-release lamotrigine was approximately 21% lower than immediate-release lamotrigine in subjects receiving enzyme-inducing AEDs. However, a reduction in exposure of up to 70% was observed in some subjects in this group when they switched to LAMICTAL XR. Therefore, doses may need to be adjusted in some patients based on therapeutic response.
Table 6: Steady-State Bioavailability of LAMICTAL XR
Relative to Immediate-Release Lamotrigine at Equivalent Daily Doses (Ratio of
Extended-Release to Immediate-Release 90% CI)
| Concomitant Antiepileptic Drug | AUC(0-24ss) | Cmax | Cmin |
| Enzyme-inducing antiepileptic drugsa | 0.79 (0.69, 0.90) |
0.71 (0.61, 0.82) |
0.99 (0.89, 1.09) |
| Valproate | 0.94 (0.81, 1.08) |
0.88 (0.75, 1.03) |
0.99 (0.88, 1.10) |
| Antiepileptic drugs other than enzyme-inducing antiepileptic drugsa or valproate | 1.00 (0.88, 1.14) |
0.89 (0.78, 1.03) |
1.14 (1.03, 1.25) |
| aEnzyme-inducing antiepileptic drugs include carbamazepine, phenytoin, phenobarbital, and primidone. |
Dose Proportionality
In healthy volunteers not receiving any other medications and given LAMICTAL XR once daily, the systemic exposure to lamotrigine increased in direct proportion to the dose administered over the range of 50 to 200 mg. At doses between 25 and 50 mg, the increase was less than dose proportional, with a 2-fold increase in dose resulting in an approximately 1.6-fold increase in systemic exposure.
Distribution
Estimates of the mean apparent volume of distribution (Vd/F) of lamotrigine following oral administration ranged from 0.9 to 1.3 L/kg. Vd/F is independent of dose and is similar following single and multiple doses in both patients with epilepsy and in healthy volunteers.
Protein Binding
Data from in vitro studies indicate that lamotrigine is approximately 55% bound to human plasma proteins at plasma lamotrigine concentrations from 1 to 10 mcg/mL (10 mcg/mL is 4 to 6 times the trough plasma concentration observed in the controlled efficacy trials). Because lamotrigine is not highly bound to plasma proteins, clinically significant interactions with other drugs through competition for protein binding sites are unlikely. The binding of lamotrigine to plasma proteins did not change in the presence of therapeutic concentrations of phenytoin, phenobarbital, or valproate. Lamotrigine did not displace other AEDs (carbamazepine, phenytoin, phenobarbital) from protein-binding sites.
Metabolism
Lamotrigine is metabolized predominantly by glucuronic acid conjugation; the major metabolite is an inactive 2-N-glucuronide conjugate. After oral administration of 240 mg of 14C-lamotrigine (15 μCi) to 6 healthy volunteers, 94% was recovered in the urine and 2% was recovered in the feces. The radioactivity in the urine consisted of unchanged lamotrigine (10%), the 2-N-glucuronide (76%), a 5-N-glucuronide (10%), a 2-N-methyl metabolite (0.14%), and other unidentified minor metabolites (4%).
Enzyme Induction
The effects of lamotrigine on the induction of specific families of mixed-function oxidase isozymes have not been systematically evaluated.
Following multiple administrations (150 mg twice daily) to normal volunteers taking no other medications, lamotrigine induced its own metabolism, resulting in a 25% decrease in t½ and a 37% increase in CL/F at steady state compared with values obtained in the same volunteers following a single dose. Evidence gathered from other sources suggests that self-induction by lamotrigine may not occur when lamotrigine is given as adjunctive therapy in patients receiving enzyme-inducing drugs such as carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation.
Elimination
The elimination half-life and apparent clearance of lamotrigine following oral administration of immediate-release lamotrigine to adult subjects with epilepsy and healthy volunteers is summarized in Table 7. Half-life and apparent oral clearance vary depending on concomitant AEDs.
Since the half-life of lamotrigine following administration of single doses of immediate-release lamotrigine is comparable with that observed following administration of LAMICTAL XR, similar changes in the half-life of lamotrigine would be expected for LAMICTAL XR.
Table 7: Mean Pharmacokinetic Parametersa of
Immediate-Release Lamotrigine in Healthy Volunteers and Adult Subjects With
Epilepsy
| Adult Study Population | Number of Subjects | t½: Elimination Half-life (h) | CL/F: Apparent Plasma Clearance (mL/min/kg) |
| Healthy volunteers taking no other medications: | |||
| Single-dose lamotrigine | 179 | 32.8 (14.0-103.0) |
0.44 (0.12-1.10) |
| Multiple-dose lamotrigine | 36 | 25.4 (11.6-61.6) |
0.58 (0.24-1.15) |
| Healthy volunteers taking valproate: | |||
| Single-dose lamotrigine | 6 | 48.3 (31.5-88.6) |
0.30 (0.14-0.42) |
| Multiple-dose lamotrigine | 18 | 70.3 (41.9-113.5) |
0.18 (0.12-0.33) |
| Subjects with epilepsy taking valproate only: | |||
| Single-dose lamotrigine | 4 | 58.8 (30.5-88.8) |
0.28 (0.16-0.40) |
| Subjects with epilepsy taking carbamazepine, phenytoin, phenobarbital, or primidoneb plus valproate: | |||
| Single-dose lamotrigine | 25 | 27.2 (11.2-51.6) |
0.53 (0.27-1.04) |
| Subjects with epilepsy taking carbamazepine, phenytoin, phenobarbital, or primidone:b | |||
| Single-dose lamotrigine | 24 | 14.4 (6.4-30.4) |
1.10 (0.51-2.22) |
| Multiple-dose lamotrigine | 17 | 12.6 (7.5-23.1) |
1.21 (0.66-1.82) |
| aThe majority of parameter means determined in each study
had coefficients of variation between 20% and 40% for half-life and CL/F and
between 30% and 70% for Tmax. The overall mean values were calculated from
individual study means that were weighted based on the number of
volunteers/subjects in each study. The numbers in parentheses below each
parameter mean represent the range of individual volunteer/subject values
across studies. bCarbamazepine, phenytoin, phenobarbital, and primidone have been shown to increase the apparent clearance of lamotrigine. Estrogen-containing oral contraceptives and other drugs, such as rifampin and protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir, that induce lamotrigine glucuronidation have also been shown to increase the apparent clearance of lamotrigine. |
|||
However, we will provide data for each active ingredient


