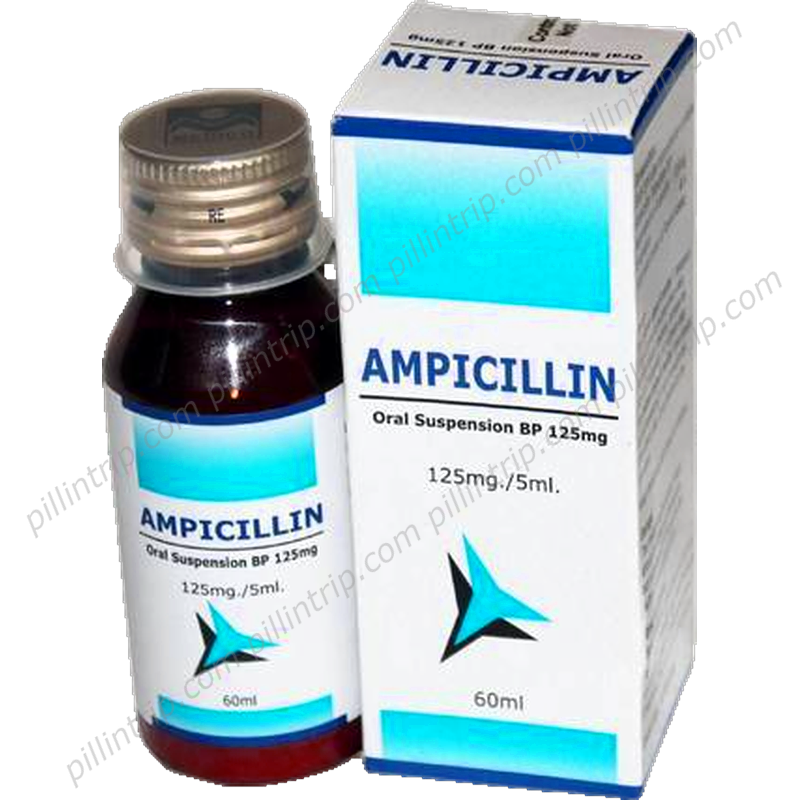
Principen (Ampicillin)
Quick links to important sections
Principen (Ampicillin)
Medically reviewed
Last updated on 10/8/2025
Attention! Information on this page is intended only for medical professionals! Information is collected in open sources and may contain significant errors! Be careful and double-check all the information on this page!
What Principen (Ampicillin) treats: main uses and benefits
What is Principen (Ampicillin)?
Attention! Always consult to a doctor or pharmacist before using pills or medicines.
Available in countries:
Equivalent of Principen (Ampicillin) found in:
 Portugal
Portugal Russia
Russia Mexico
Mexico Colombia
Colombia Cyprus
Cyprus India
India Czech Republic
Czech Republic Georgia
Georgia Lebanon
Lebanon Bosnia & Herzegowina
Bosnia & Herzegowina Israel
Israel Canada
Canada Denmark
Denmark Argentina
Argentina Belgium
Belgium Norway
Norway Thailand
Thailand Switzerland
Switzerland Indonesia
Indonesia South Korea
South Korea Bulgaria
Bulgaria Turkey
Turkey Malasia
Malasia Finland
Finland Bangladesh
Bangladesh China
China Brasil
Brasil Ukraine
Ukraine Vietnam
Vietnam Spain
Spain Phillipines
Phillipines United Kingdom
United Kingdom Costa Rica
Costa Rica Tunisia
Tunisia Greece
Greece Sweden
Sweden Macedonia
Macedonia Hong Kong
Hong Kong Serbia
Serbia Japan
Japan Kenya
Kenya South Africa
South Africa Oman
Oman Germany
Germany Belize
Belize Poland
Poland Egypt
Egypt Italy
Italy Taiwan
Taiwan Austria
Austria France
France Venezuela
Venezuela Malta
Malta Ecuador
Ecuador Netherlands
Netherlands Australia
Australia Chile
Chile Lithuania
Lithuania Latvia
Latvia Trinidad & Tobago
Trinidad & Tobago Bahrain
Bahrain Peru
Peru Hungary
Hungary New Zealand
New Zealand Slovakia
Slovakia Myanmar
Myanmar Singapore
Singapore Pakistan
Pakistan Croatia (Hrvatska)
Croatia (Hrvatska) Estonia
Estonia Romania
Romania Slovenia
Slovenia